China's Vaccine road tripWith few COVID-19 cases at home, Chinese vaccinemakers have had to test the worth of their candidates abroad and four are in efficacy trials in 15 countries.
The two other Chinese players, Sinovac Biotech and China National Biotec Group (CNBG)—a subsidiary of one of the world’s largest vaccinemakers, the state-owned Sinopharm—are taking a different approach: vaccinating people with the whole, “killed” virus. This requires no sophisticated protein or RNA design or genetic engineering: Scientists simply inactivate the virus with a chemical (beta propiolactone) and mix it with an adjuvant (alum) that effectively puts the immune system on full alert by irritating it. In theory, such vaccines can produce broader antibody and T cell responses, because they contain the full set of viral proteins, rather than a single one such as spike. And unlike mRNA vaccines, which have to be stored at subzero temperatures, inactivated viruses requires no more than ordinary refrigeration. Read the full article here. By John Cohen
0 Comments
11/20/2020 China has given almost a million people experimental Covid vaccine, says companyRead NowSinopharm chairman claims there has not been a single case of infection after inoculation of officials, students and workers heading overseas
Almost a million people in China have taken an emergency Covid-19 vaccine that is still in its testing phase, the company that developed the vaccine has said. Chinese authorities released the vaccine, developed by China National Pharmaceutical Group (Sinopharm), to select groups of people in July including Chinese government officials, students, and workers travelling overseas, before the vaccines had been proven to work. Sinopharm’s claims, made in an interview with chairman Liu Jingzhen posted on WeChat, did not specify which of its vaccines had been given, but said the individuals had travelled to more than 150 countries around the world and “there has not been a single case of infection after inoculation”. “Only individual patients have had some mild symptoms,” he said. Sinopharm’s two vaccine candidates are among five Chinese candidates undergoing international clinical trials because the domestic prevalence of the disease is so low. At least three – all inactivated vaccines from Sinopharm and Sinovac – have been approved for emergency use outside the clinical trials, and some local governments have reportedly allowed residents to take the Sinovac vaccine. In September, the United Arab Emirates was the first country outside China to approve emergency use of Sinopharm’s vaccine. Read the full article here Additional reporting by Lillian Yang 11/20/2020 Almost a million people have been given an experimental Chinese coronavirus vaccine, pharmaceutical giant claimsRead NowBy Ben Westcott and Sophie Jeong, CNN
Hong Kong (CNN) Almost a million people have been given an experimental coronavirus vaccine developed by Sinopharm as part of an emergency-use program authorized by Beijing, the Chinese pharmaceutical giant's chairman said. No serious adverse effects have been reported from vaccine recipients so far, Sinopharm said Wednesday in an article on social media platform WeChat, citing Chairman Liu Jingzhen. "In emergency use, we now have used it on nearly a million people. We have not received any reports of serious adverse reaction, and only a few have some mild symptoms," Liu said. Liu said the vaccine had been given to Chinese construction workers, diplomats, and students who have gone to more than 150 countries around the world during the pandemic -- and none of them has reported an infection. He said on November 6 that there were 56,000 people who had received emergency vaccinations and then gone overseas. "For example, a transnational company has 99 employees in one of its overseas offices, of whom 81 were vaccinated. And later, an outbreak broke out in the office, 10 of the 18 people who were not vaccinated were infected and none of those vaccinated were infected," he said. He said that separately, Sinopharm had carried out Phase 3 clinical trials involving almost 60,000 people in 10 countries -- including the UAE, Bahrain, Egypt, Jordan, Peru, and Argentina. CNN has not seen the results of these trials yet. Read the full article here. China’s vaccines are also very effective: head of China’s CDCBy Hu Yuwei and Leng Shumei Source: Global Times 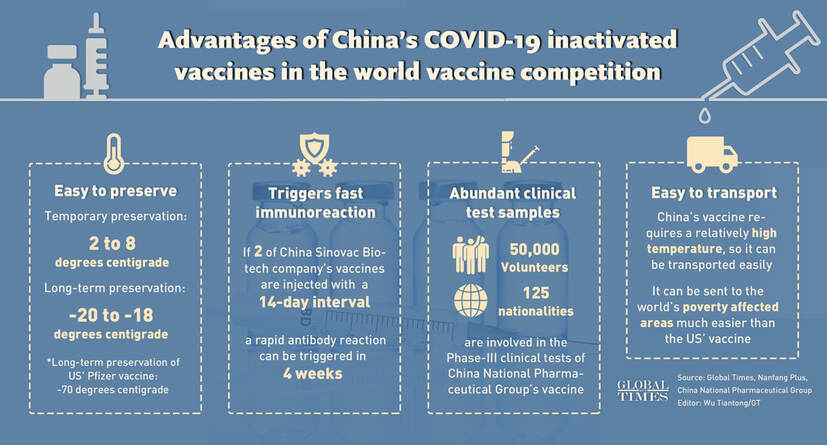 Advantages of China’s COVID-19 inactivated vaccines in the world vaccine competition Infographic: GT Chinese vaccine makers said that China's full-throttled development of COVID-19 vaccines remains in the lead among a roster of effective vaccines, although some Western media and politicians have questioned China for delays in releasing late-stage trial data after US drug makers Pfizer and Moderna's successes. The fact is, however, at least three out of five types of Chinese vaccines against the coronavirus have entered final stage trials, with the latest beginning phase-III trials in central China's Hunan Province on Thursday.
Read the full article here A Covid-19 vaccine developed in China has shown success in mid-stage trials, researchers say.There are several vaccines being developed in China, some of which are already being administered. According to the researchers, the Sinovac Biotech vaccine led to a quick immune response during trials with around 700 people. Promising details, but we should waitAnalysis by Pallab Ghosh, Science correspondent, BBC
Following the announcement of not one but two successful potential vaccines for coronavirus in the space of a week, attention is understandably focussed on the minutiae of the latest developments. Under normal circumstances the results of phase 1 and phase 2 trials of a drug would not raise an eyebrow outside the lab. These stages are largely to check if the pharmaceutical is safe and might be effective to try out in more expensive-to-run phase 3 trials. This is what Sinovac has announced. Its vaccine is different from the ones announced by Pfizer/BionTech and Moderna in that it has been developed using more traditional methods. It uses a chemically inactivated version of the virus. The manufacturers highlight the fact that their vaccine gives a "quick" response in the trials - developing virus-fighting antibodies within 14 days of receiving a dose. This feature would make it suitable for emergency use, they say, during an outbreak or for healthcare workers. All promising stuff, but we should wait to see the results of the larger scale trials currently under way in Brazil, Indonesia and Turkey before drawing any firm conclusions about its effectiveness. And even then, just like the phase 3 results that have been and will soon be announced, they should be taken in with a healthy dose of caution. Researchers won't know how effective any of them will be in the long term until they start being rolled out in the general population. The development of vaccines does not mark the end of the pandemic, but the beginning of a long and slow return to normality. Read the full article here Not ready except for "emergency use"China has four experimental vaccines developed by three companies now going through phase III human trials and safety testing before they can be approved for commercial use. Sinopharm and Sinovac, the two front-runners, have both stressed they are not ready to deploy their vaccines commercially.
"The vaccines developed by Sinopharm are in the final sprint, the last kilometer in a long march," said Liu Jingzhen, the company's chairman, said at a government press conference in October. In reality, their vaccines had already been deployed months earlier this summer for "emergency use" and shot into the arms of people that China deems vulnerable to COVID-19, including front-line medical workers and critical service providers in large cities. Sinovac has already set up several vaccination sites in China's coastal Zhejiang province where several hundred vaccine doses each day are sold first come first served for about RMB400 ($60) in out of pocket costs. Other countries are following suit; the Philippines says it will vaccinate up to 9 million people with Sinovac's product. The Brazilian state of Sao Paolo is conducting advanced human trials for Sinovac and intends to buy the vaccine once approved. Read the full article here |
Details
Categories
All
Archives
June 2024
|
Practical Solutions for complex and urgent global medical problems
© 2024 Refana Inc







 RSS Feed
RSS Feed